Objectives
Define processes and set standards for in silico trials and their integration in the drug development chain.
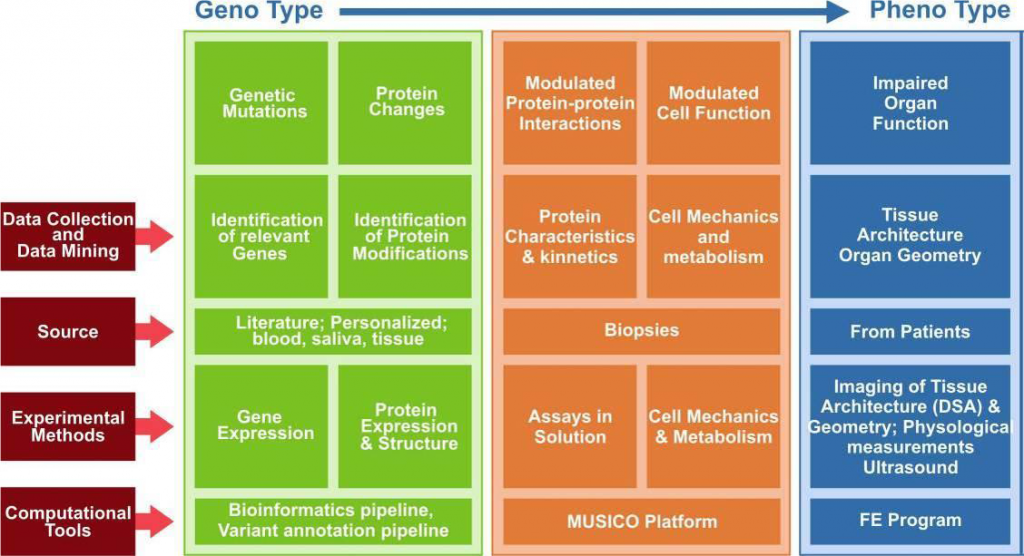
Identify and relate specific gene or genes to protein structural, kinetics and functional changes for specific drugs interaction.
Perform experimental determination of material characteristics of heart muscles, from for example LV wall, along with biomechanical parameters, gene expression, protein kinetics and the whole organ functional images/movies.
Perform clinical studies for determination of risk factors associated with drugs interaction for DCM, HCM, RCM and ARVC on a clinically relevant number (200-300) of patients from 4 clinical partners.
Develop software tools for collection and analysis of patient-specific data and development of patient-specific models for monitoring and assessment of patient condition from current through the progression of disease.
Provide a library of virtual patients for re-use in pre- and post-competitive testing of drugs using data mining approach.
Deliver a software platform for in silico efficacy and safety tests and integrate the platform in the drug development chain to support design and regulatory approval processes.
Evaluate the in silico trial platform in representative scenarios to demonstrate the capabilities of this approach and propose measures of validation for in silico trials.
